
▶ Previous Artlcle : #1-1. Utilization of Selenium Injection
Chemical Understanding of Selenium
Selenium is a chemical element with atomic number 34 on the periodic table of the elements and belongs to Group 6 of the periodic table along with oxygen and sulfur (Figure 1).
Oxygen, sulfur, and selenium are all atoms belonging to the sixth group of the periodic table and have six electrons revolving in the outermost shell of the atom.
With an odd number of only two electrons, these atoms in group 6 make molecules with a characteristic form of combination (Figure 2).
[Advertisement] MAGNUM(Q-switched Nd:YAG Laser) – Manufacturer: (www.i-dana.com)]
Therefore, selenium is used in the same way as sulfur belonging to group 6, like hydrogen sulfide (hydrogen selenide), sulfur dioxide (selenium dioxide), sulfurous acid (selenious acid), and sulfuric acid (selenic acid) according to combination type.
Selenium is sometimes combined in the place where sulfur would have been (Figure 3).
Selenium is classified into inorganic selenium, organic selenium, and selenoprotein in terms of chemical structure.
As for inorganic selenium, it is divided into five categories.
One is selenium (Se2) and another is hydrogen selenide (H2Se). Hydrogen selenide is important because it is a basic substance that forms selenoproteins in the body.
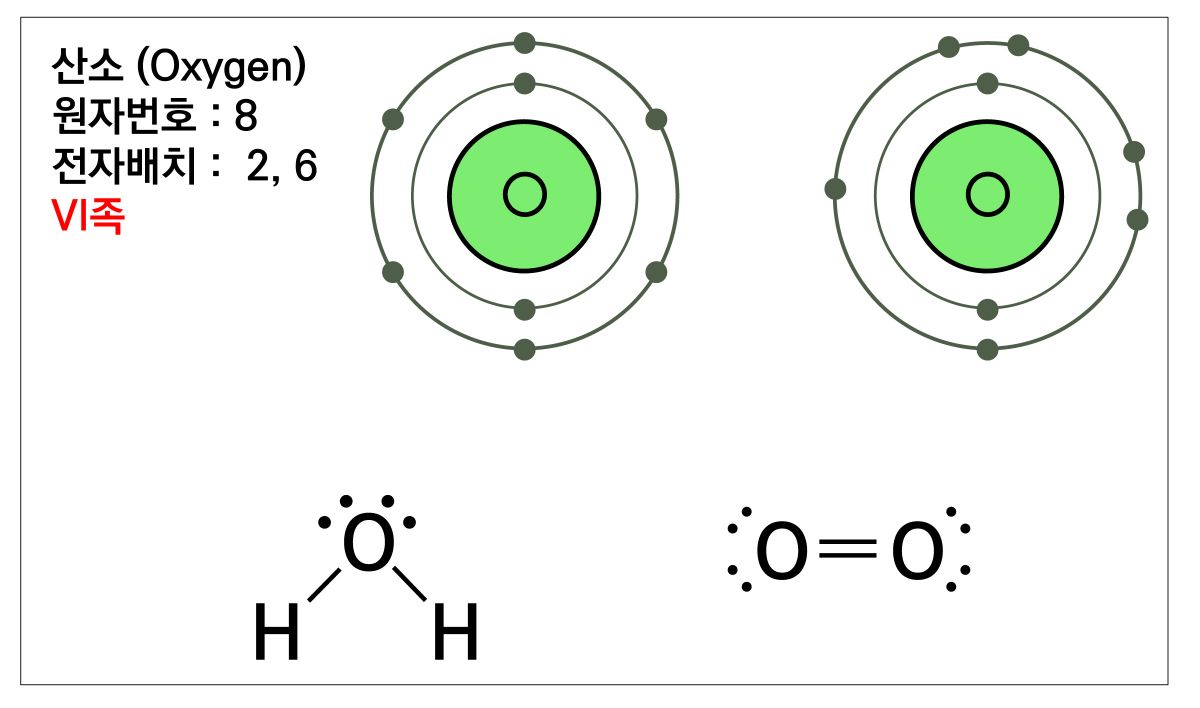
Figure 2. Combination Structure of Oxygen in Group 6. Atomic number 8, Electron configuration 2,6, Group VI.
-To be continued




















