Tyrosinase Inhibitors
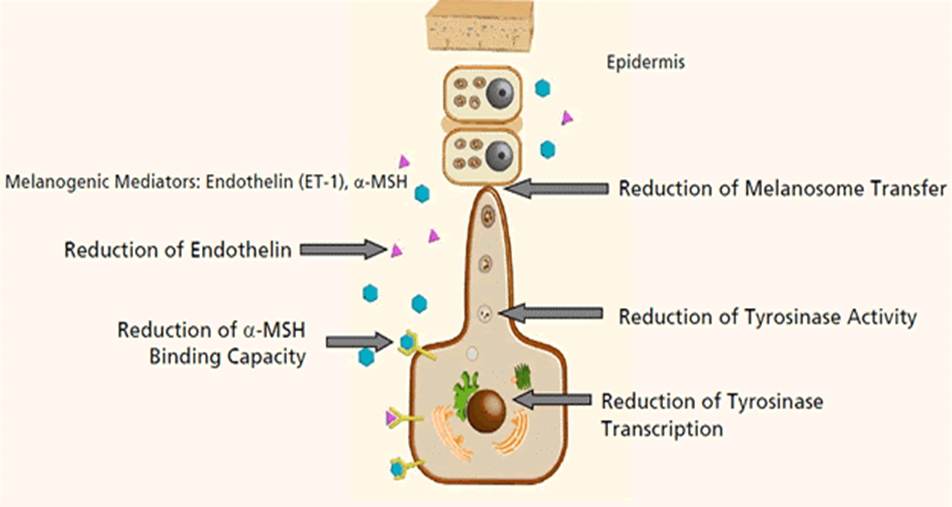
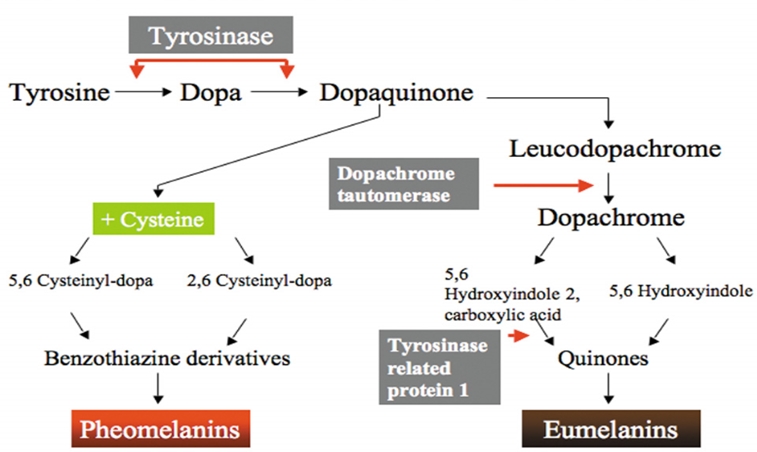
Tyrosinase is a rate-limiting enzyme of melanogenesis by epidermal melanocytes. Some whitening products currently marketed contain ingredients that inhibit tyrosinase and reduce melanogenesis.
Hydroquinone
Hydroquinone (HQ) is naturally found in vegetables, fruits, grains, coffee, tea, beer and wine, etc. It is one of the most common ingredients of whitening cosmetic products[i]. For many years, it has also been used as a therapeutic agent in melasma and hyperpigmentation. HQ inhibits tyrosinase and has cell toxicity toward melanocytes which leads to depigmenting action[ii]. This affects DNA and RNA synthesis and reversibly inhibits cellular metabolism. Moreover, HQ inhibits tyrosinase activity by up to 90%[iii]. HQ can be used alone or in combination with tretinoin, GA, kojic acid, or azelaic acid, etc.[iv]
Topical use of HQ initially causes skin irritation or sensitivity but when used continuously for at least 6 weeks, these symptoms disappear. Safety of HQ has been an issue for many years. In 2000, Europe banned HQ in general cosmetics. HQ is a by-product of benzene and has been extensively studied due to its possible carcinogenicity. Some studies have shown that high dose HQ led to systemic absorption and development of leukemia in rats. However, hydroquinone has been marketed for over 4 decades and there have not been any reports of it causing cancer in humans. Laboratory workers that are exposed to HQ have a high risk of pigmented lesions of the eyes and permanent cornea damage, causing FDA to approve HQ as a topical agent only. Topical application can also cause side effects such as exogenous ochronosis[v]. Topical use of HQ in ochronosis can cause asymptomatic blue-black macules (a permanent form of hyperpigmentation) in the site of application. This side effect can often be caused by long-term exposure to at least 4% HQ but ochronosis was reported with 2% HQ as well[vi]. This may be due to the fact that HQ’s inhibition of homogentisic acid oxidase leads to local accumulation of homogentisic acid and ochronotic pigments[vii]. Only 30 cases of exogenous ochronosis have been reported in Caucasians but it can be more prevalent in those with darker skin.
[Advertisement] MAGNUM(Q-switched Nd:YAG Laser) – Manufacturer: (www.i-dana.com)]
Other side effects of HQ include redness or discoloration of finger nails which improve when HQ is discontinued. Low-dose HQ has a much lower frequency of side effects. HQ is an excellent whitening agent but its use has been limited due to various side effects. However, thanks to better understanding of various mechanisms of pigmented lesions, different topical agent ingredients have been developed; 4-ethoxybenzaldehyde (anti-inflammatory, PGE2 suppressor), licorice extract (tyrosinase inhibitor), tetrahexyldecyl ascorbate (antioxidant), nyacinamide (melanosome transport inhibitor), ethyl linoleate (tyrosinase inhibitor), hexylresorcinol (tyrosinase inhibitor), and retinol (tyrosinase transcription inhibitor)[viii].
[i] DeCaprio AP. The toxicology of hydroquinone-relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999 May;29(3):283-330.
[ii] Penney KB, Smith CJ, Allen JC. Depigmenting action of hydroquinone depends on disruption of fundamental cell processes. J Invest Dermatol. 1984 Apr;82(4):308-10.
[iii] Nordlund JJ. Postinflammatory hyperpigmentation. Dermatol Clin. 1988 Apr;6(2):185-92.
[iv] Guevara IL, Pandya AG. Melasma treated with hydroquinone, tretinoin, and a fluorinated steroid. Int J Dermatol. 2001 Mar;40(3):212-5.
[v] Lawrence N, Bligard CA, Reed R, Perret WJ. Exogenous ochronosis in the United States. J Am Acad Dermatol. 1988 May;18(5 Pt 2):1207-11
[vi] Barrientos N, Ortiz-Frutos J, Gómez E, Iglesias L. Allergic contact dermatitis from a bleaching cream. Am J Contact Dermat. 2001 Mar;12(1):33-4.
[vii] Kramer KE, Lopez A, Stefanato CM, Phillips TJ. Exogenous ochronosis. J Am Acad Dermatol. 2000 May;42(5 Pt 2):869-71.
[viii] Makino ET, Mehta RC, Banga A, et al. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol. 2013 Mar;12(3):s16-20.
-To be continued-