▶ Previous Artlcle : #15-1. Cell therapy with human allogeneic keratinocytes
Kaloderm is Korea’s first allogeneic cell therapy developed from culturing keratinocytes harvested from the foreskin a Korean neonatal. Culture allows production of tens of thousands of Kaloderm products without having to recruit more donors. This allows consistent quality and excellent availability as it can be easily stored and used immediately as needed, unlike cultured autologous skin. One product (25cm2, Vaseline gauze 5.0cm×5.0cm) contains 9×106 human keratinocytes. However, Kaloderm requires cryopreservation (-60℃ for 24 months, 15℃ for 3 months) and needs to be defrosted for 5-10 minutes at room temperature before application. Also, the epidermal basement membrane should come in contact with the wound (no distinction of top and bottom of the sheet).
[Advertisement] MAGNUM(Q-switched Nd:YAG Laser) – Manufacturer: (www.i-dana.com)]
In the 2003 clinical study on Kaloderm applied in patients with deep second degree burn, the treatment period was only 3-4 days. In terms of safety, no special adverse drug reactions or serious adverse events occurred except for pain and pruritus in the burn sites. In 2009, 59 patients with diabetic foot ulcer were treated with Kaloderm in a clinical study. A 12-week efficacy study after treatment showed a high cure rate of 31% and the mean treatment period was also 22 days shorter.
Allogeneic keratinocyte sheets have a significant advantage over conventional treatments in terms of shorter healing time, quicker epithelialization and scar reduction. They are expected to be more widely applied to deep abrasive wounds and scars, etc. in the near future.
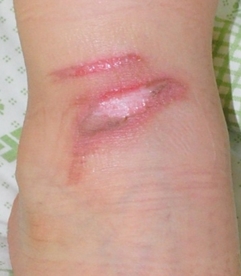
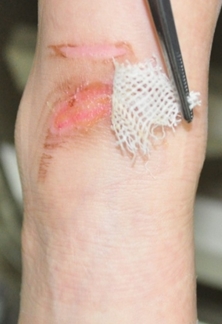
Figure 2. Clinical case of allogeneic keratinocyte application. Deep second degree burn in the ankle of a 5-year-old child (left: before application. right: healed wound 7 days after treatment).
References
1) Han SK, You HJ. Wound coverage using advanced technology in Korea. J Korean Med Assoc 2011; 54 (6): 594-603
2) You HJ, Han SK, Lee JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes–a pilot study Wound Repair Regen. 2012; 20 (4):491-9.
3) Khachemoune A, Bello YM, Phillips TJ. Factors that influence healing in chronic venous ulcers treated with cryopreserved human epidermal cultures. Dermatol Surg. 2002; 28 (3):274-80.
4) Bolívar-Flores YJ, Kuri-Harcuch W. Frozen allogeneic human epidermal cultured sheets for the cure of complicated leg ulcers. Dermatol Surg. 1999; 25 (8):610-7.
-To be continued-
▶ Next Artlcle : #16-1. Understanding and Clinical Application of basic Fibroblast Growth Factors (bFGF)





















