
Dr. Jung-whan Baek (H Plastic Surgery)

Figure 3. Customized plate manufacturing.
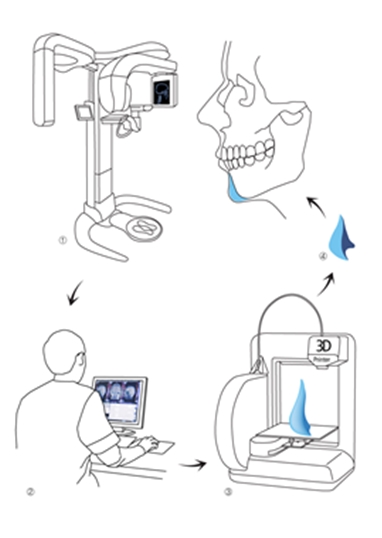
As shown in <Figure 3>, the key advantage of 3D printing is the ability to manufacture an intricate, three-dimensional, and customized plate before surgery. The biggest challenge to this technology is not the lack of technology but the restricted choice of materials. Only a few metals and synthetic polymers can be used in 3D printing. And the number of materials decreases further when limiting to only those that are approved. Even one material had been approved for medical use, a new application process is needed when it is used in 3D printing due to a change in manufacturing process. Clinical study data, toxicity data, and biocompatibility data are needed for approval. However, there are no standards for assessing 3D printing materials and traditional criteria are used in the approval process. This systemic inadequacy seriously hampers application of this new technology.
Terminology and fabrication process of customized implant
Customized implants are probably the most useful in the field of plastic surgery. I believe this new technology has great potential in both aesthetic and reconstructive plastic surgery. While I was researching various ways to apply this new technology to plastic surgery, I felt the need for clearer terminology and have coined below terms for medical 3D printing.
1. 3D Fit
In the past, surgical facial contouring mainly involved resection or shifting of bones. Unlike soft tissues like fat or skin, bone tissues could not be harvested from a donor site for grafting due to the high risk of donor site dysfunction and disfiguration. The term ‘3D Fit’ refers to surgical methods that improve on the limitations of traditional methods. 3D Fit encompasses three-dimensional simulation, modeling and manufacturing of a cutting guide for preventing excessive bone resection and customized bone replacement for excessive resection of facial bone.
2. 4D Fit
4D Fit is a surgical method that improves upon 3D Fit. It combines 3D printing with biochemical and tissue engineering for providing biocompatible implant materials. For example, tissue replacement using scaffold and stem cells, biocompatible surface coating, and varying printing materials can lead to development of biocompatible implant or replacement materials.
3. Facial sculpting
Previous facial contouring methods focused on resection. On the other hand, the concept of sculpting can be applied to plastic surgery thanks to the development of 3D printing. ‘Facial sculpting’ refers to all new surgical methods ranging from cutting to sculpting facial features.
Along with terminology, I have conceptualized processes for manufacturing and implanting 3D Fit products as below.
1. 3D modeling followed by manual fabrication of temporary implant, mold, and final implant
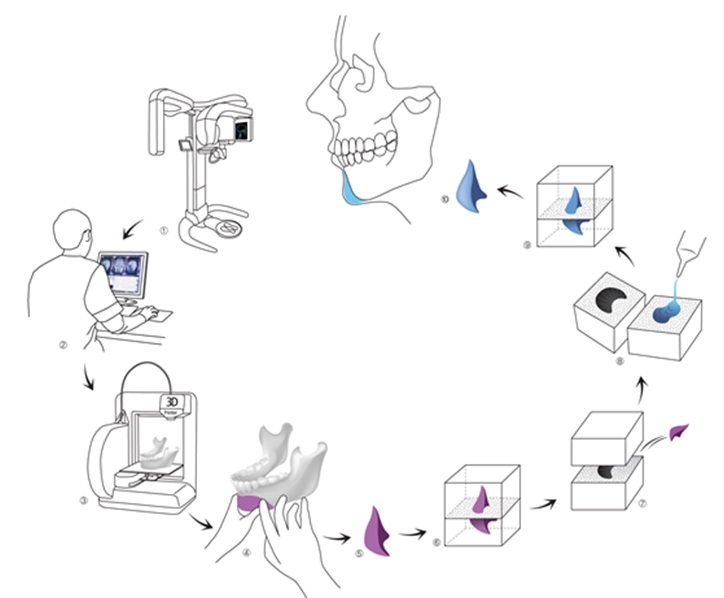
a. 3D modeling of human bone using CT image
b. Creation of 3D implant model based on modeling
c. Creation of mold using implant model
d. Fabrication of final implant(silicone etc.) using mold
e. Sterilization of the final implant(EO or gamma sterilization)
f. Surgical engraftment
I devised this process for customizing the silicone implant, one of the most commonly used implant type in plastic surgery. It ensures safety of the material but has the disadvantage of a complicated manufacturing process.
2. 3D modeling and manual fabrication of implant in surgery
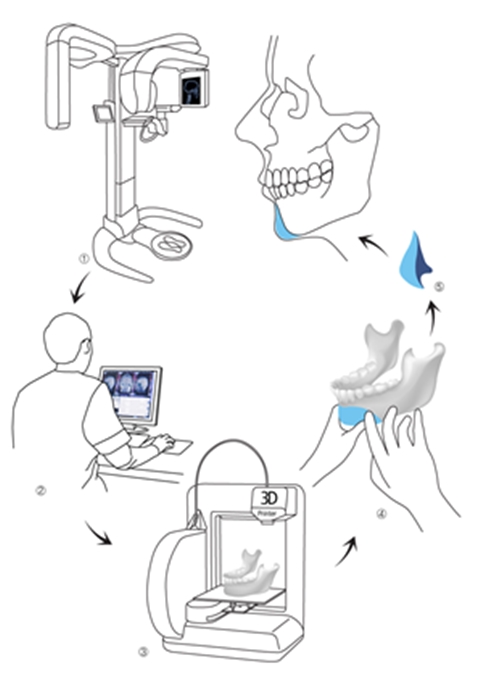
a. 3D modeling of human bone using CT image
b. Sterilization of 3D model
c. Fabrication of final implant(bone cement etc.) during surgery using 3D model
d. Placement of implant in patient after hardening
This may be the most practical method of producing bone replacement and has simper fabrication process compared to the first method. However, manual fabrication of the implant does not assure consistent quality due to varying degrees of the surgeon’s experience and dexterity.
3. 3D modeling & fabrication of final implant
a. Creation of implant using computer imaging, simulation, design
b. Sterilization of final implant(EO or gamma sterilization)
c. Placement of final implant in patient in surgery
All minor processes of this method is automated. However, it requires a lot of medical knowledge for the modeling technician and the surgical outcome is determined by the modeling technician, not the surgeon. This method may not be widely applicable as very few materials are both 3D printable and implantable in the human body.
Problems to clinical application and possible solutions
Printer and material limitations
Applying 3D printing in medicine has many challenges. First, there are only a handful of materials that can be implanted in the human body. That is, there are only a few materials that can be used in 3D printed implants. Currently, only titanium and PEKK(poly ether ketone ketone) can be 3D printed. However, these materials require a costly printer(USD 1-2.5 million) as well as special approval from the health authorities in Korea. Therefore, the third method of producing implants is currently not feasible.
The first and second methods are more practical. In the first method of creating a mold, non-toxic materials can be printed. However, the mold used in 3D printing is not as rigid as the metal mold and this is a major limitation. Even if the right material is found, a temporary implant should be fabricated in the same process described in the second method, which adds an unnecessary step. To avoid this, the mold itself can be 3D printed but in this case, the implant form should be accurately simulated on the computer. This is not feasible as a software sophisticated enough to do such simulation has not been developed yet.
Therefore, I have decided that the second method would be the most applicable in clinical practice. I have narrowed down the choice of printers to the following two; 3D Systems’s Projet and Stratasys’s Objet. These printers can use materials that can be allowed in the operation room after sterilization. As the 3D systems’s model passed ISO 109933 and USP Class IV in terms of the build size, resolution and surface finish, it was selected as the best choice.
[Advertisement] FCR® (Fractional Prickle CoralCalcium Regentron) – Manufacturer: (www.illglobal.com)]
-To be continued-




















