The CaHA dermal filler is a gel carrier consisting of 30% calcium hydroxylapatite microspheres, water, glycerin and carboxymethylcellulose. Recent studies report that the CaHA filler treatments does not cause calcification or osteogenesis and CaHA is dissolved and excreted through metabolic processes.
One special characteristic of this filler is that it promotes collagenesis after injection. This is observable in radiologic or CT examinations as well as FDG=PET or MRI up to 12 months after injection. As the particle size is small at about 25~45μm, they avoid being engulfed by macrophages, which enables them to act as a continuous biostimulator. In other words, the desired volume is maintained through continued collagenesis. Recent studies report that it generates elastin more effectively than traditional hyaluronic acid (HA) fillers.
HELIOSⅡ/LOTUSⅡ/HYPERION – Manufacturer: LASEROPTEK(www.laseroptek.com)
Efficacy does not solely depend on the physical properties of the filler. Patient’s tissue characteristics, age, degree of photo-aging, injection site or depth, doctor’s skills, post-treatment patient conditions, sex, and immunity, etc. also affect the duration of the filler’s effect.
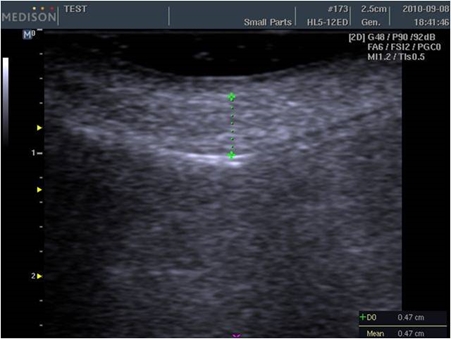
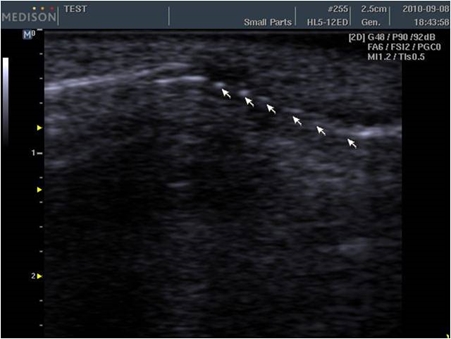
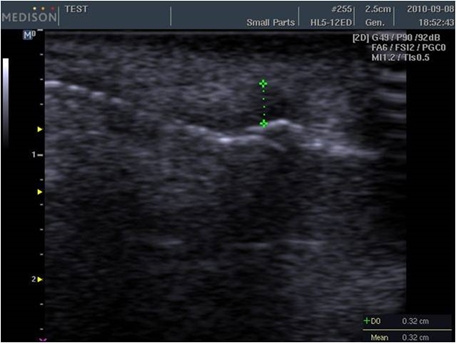
Side effects of CaHA filler
Injection-related side effects include bruising, edema, and pain, etc. with very rare cases of tissue necrosis. However, some doctors advise against mixing lidocaine and epinephrine to detect early ischemic changes and prevent necrosis. On the other hand, some doctors argue that mixing lidocaine with epinephrine reduces pain and bruising.
A 47-month evidence level II study enrolling 113 patients reported a 90% satisfaction rate. Following multiple injections in the face (nasolabial folds, perioral lines, cheeks, acne scars, tear trough depressions, and pre-jowl sulci), minor side effects were reported in 8 patients, all of which were resolved in one month after treatment. Three patients presented bruises, and 2 experienced nongranulomatous submucosal nodules after lip injection. The last 3 presented inflammation and edema. The submucosal nodules of the lips were treated with steroid injections. One study reported the incidences of nodules as 0.001% for CaHA filler, 0.04-0.4% for HA fillers, 0.12% for silicone, 0.2-1% for poly L-lactic acid, and 1.51% for polymethyl methacrylate. According to another report, filler treatments are not followed by dyspigmentation, keloid formation, or hypertophic scar formation. Systemic reactions have not been reported other than one case of systemic reaction following vocal cord injection.
-To be continued-