▶ Previous Artlcle : #10-2. Biological Dressing I
Cultured Epithelial Homograft (CEH)
CEH differs from cultured epithelial autograft (CEA) in that it does not involve permanently engrafting proliferating stem cells onto the lesion site. In CEH therapy, temporary transplantation of keratinocytes provide various growth factors or cytokines necessary for wound healing and they in turn stimulate cells surrounding the wound to promote the healing process. In other words, CEH works as a catalyst that accelerates the healing process and re-epithelialization.
[Advertisement] MAGNUM(Q-switched Nd:YAG Laser) – Manufacturer: (www.i-dana.com)]
Therefore, CEH is not permanently engrafted to remain as part of the skin, but has the advantage of speedy apply compared to CEA which requires at least two weeks for cell culture.
Cultured skin homograft does not cause immune responses. For example, Langerhans cells in culture do not attach to the vessel and are removed. Thus, culture with duration exceeding seven days does not contain Langerhans cells. As Langerhans cells expressing class II antigen are absent, Class II HLA-DR antibodies are not expressed and therefore no rejection occurs despite histological incompatibility. However, CEH with identical HLA(Human Leukocyte Antigen) type are eventually rejected in the human body. When CEH with different blood group antigen from MHC was grafted onto grade III burn site and donor site, it was rejected in 14.5 days.
Compared to transplantation of cultured skin homograft of cadaver skin, rejection was drastically delayed by 4-5 days and hence offered improved safety. This clinical rejection takes place in the form of complete dissolution of the cultured skin. Transplanted homograft keratinocytes were shown to be completely replaced by recipient cells within 2-4 weeks or 6 months in a DNA analysis. Therefore, CEH applied to a wound does not survive as the cultured autograft after transplantation but greatly contributes to the wound healing process.
Kaloderm, cellular therapy using CEH
In 2003, a clinical study approved by Ministry of Food and Drug Safety(MFDS) of Korea showed that Kaloderm was effective in wound treatment with drastic reduction in scar formation and healing time. It earned MFDS marketing authorization in 2005. Subsequently, a re-examination conducted over six years proved its efficacy and safety and Kaloderm earned re-examination approval in 2011. In addition, Korea is the only country that provides insurance coverage for Kaloderm although it is limited to some indications.
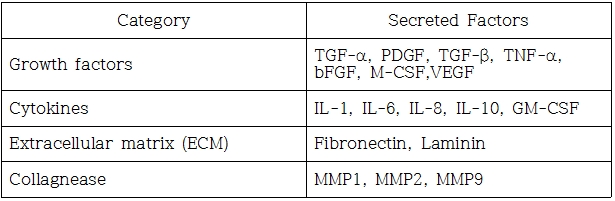
Fig1. Secreted Factors of Kaloderm
-To be continued-
▶ Next Artlcle : #11-2. Biological Dressing Ⅱ





















