▶ Previous Artlcle : #8-2. Wound Healing Process
Growth Factors
Growth factors are polypeptide molecules that oversee the growth, differentiation and metabolism of cells during the three phases of wound healing. Despite in very small amounts, growth factors play a big role in local wound healing. They also induce specific responses by a receptor-mediated signal transduction pathway through interactions with cell surface specific receptors.
Actions of growth factors include cellular proliferation, chemotaxis, haptotaxis, angiogenesis, protein expression, and enzyme production, etc. Although the roles of individual growth factors during normal human wound healing have not been fully understood, their actions have been clarified in in vitro settings. Growth factors were termed according to tissue origin (e.g. PDGF), biologic action (e.g. TGF) or the type of cells they act on (e.g. EGF). They act on adjacent cells (paracrine function), cells producing growth factors (autocrine function), or inside producing cells (intercrine function). Some growth factors are transported through plasma to attach to large carrier proteins and are released as endocrine factors.
[Advertisement] Aileen plus(Long pulsed Nd:YAG Laser) – Manufacturer: FineMEC(www.finemec.net)
Wound healing is a process where a series of growth factor signals released from activated cells induce and eventually complete tissue repair in target cells. Platelets which play a crucial role in initial wound healing contain rich sources of growth factors such as PDGF, TGF, and EGF, etc. Growth factors are also chemoattractants of neutrophils, monocytes (macrophages), fibroblast, and endothelial cells. Growth factors are released into the wound by platelets but other cells, in particular macrophages, also produce growth factors that are involved in early wound healing process. The ensuing proliferation of keratinocytes are stimulated by EGF, IGF-1 (insulin-like growth factor), TGF and interleutin-1 (IL-1). Wound remodeling is known to be controlled by the collagenase production induced by EGF, TNF, IL-1, and PDGF. Therefore, all phases of wound healing are both directly and indirectly controlled by growth factors. This understanding has led to the clinical use of various growth factors in wound healing.
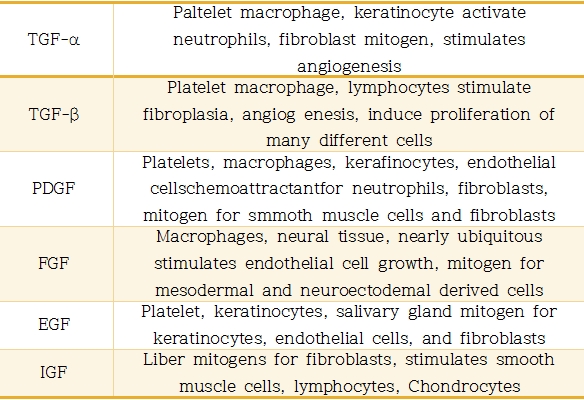
Table 1. Growth Factor Activity in Wound Healing.
TGFs
TGFs consist of two polypeptide chains. They can morph into a stimulated cell phenotype and anchorage-dependent cell stimulant that induces contact inhibition loss and anchorage in dependent growth. TGF-α has homology of amino acid sequence with EGF, however, TGF-β does not share its amino acid sequence with TGF-α, or any other known growth factor and protein. Thus, TGF-β has a structure very different from that of TGF-α.
TGF-β is a molecule with multiple functions and can induce or prevent growth and differentiation of various cells. All cells are equipped with TGF-β receptors and can therefore potentially respond to this type of growth factors. TGF-β has at least three isoforms (TGF-β1, TGF-β2, and TGF-β3). These isoforms have very similar actions and interact with the same receptor. TGF-β is known to play a crucial role in wound repair. On one hand, it stimulates synthesis of collagen, proteoglycans, elastin, and fibronectin and reduces expression of collagen and plasminogen activator on the other. Moreover, TGF-β acts like an immunosuppressive agent and controls inflammatory response. It is also an anabolic factor that induces fibrosis and angiogenesis. No clinical trials have so far examined the exact roles of TGF-α or TGF-β.
PDGF
PDGF is a polypeptide found in the α-granule of platelets and are produced by macrophages, the endothelium, and fibroblast. PDGF consists of two chains – A and B – that are held together by the disulfide bond. The two chains have 60% homology of amino acids. The B-chain is very similar to the transforming gene of the simian sarcoma virus and acute transforming retrovirus. Human proto-oncogene C-sis is similar to viral oncogene V-sis and when expressed, it bestows an autonomous growth advantage on infected cells.
There are PDGF receptors a and b. These receptors have selectivity to PDGF isoforms. PDGF is also a chemoattractant and potent mitogent to fibroblast, smooth muscle cells, and inflammatory cells. They stimulate mesenchymal cells together with TGF-β and EGF. PDGF is produced by endothelial cells, however, endothelial cells do not react to PDGF and instead stimulate nearby vascular smooth muscle cells in a paracrine fashion. On the other hand, smooth muscle cells can produce PDGF in an autocrine fashion. PDGF is considered to mediate neointimal hyperplasia following arterial injury.
PDGF has been reported to improve healing of decubitus ulcer in several clinical studies. A comparative experiment using three doses of PDGF-BB homodimer reported that PDGF reduced the area of decubitus ulcer. Administration of PDGF at 0.01, 0.1, and 1mg/cm² for 28 days in patients resulted in healing response at the highest dose and almost no response at lower doses. No toxicity was shown. Another study administered PDGF-BB at 100 or 300mg/ml in elderly patients with pressure sore for 28 days and observed significant reduction of the wound size. In addition, in patients with diabetic neurotrophic foot ulcer, comparison between PDGF-β and vehicle showed significant superior benefit of PDGF-BB over vehicle (48% vs. 28%) and no adverse reactions to PDGF. This study was the first to show the effect of single growth factor treatment on diabetic foot ulcer.
-To be continued-
▶ Next Artlcle : #9-2. Factors involved in wound healing