
In Zone E-1, a 90mm PDO thread was placed laterally 1cm apart in subcutaneous fat using a 25G needle and the threads were rotated in the same direction 10 times. In Zone E-2, a 90mm 4-0 PDO needle was placed laterally in subcutaneous fat 0.5cm apart using a 25G needle. The needle was rotated in the same direction 10 times. In Zone E-3, a 90mm 4-0 PDO thread was placed longitudinally and laterally (mesh) 0.5 cm apart using a 25G needle. The needle was twisted in the same direction 10 times.
In Zone F-1,a 90mm 4-0 PDO thread was placed laterally in the dermis 1cm apart using a 25G needle. In Zone F-2, a 90mm 4-0 PDO thread was placed laterally in the dermis 0.5cm apart using a 25G needle. In Zone F-3, a 90mm 4-0 PDO thread was placed in mesh form 0.5 cm apart in the dermis using a 25G needle.
In Zone G-1, a 90mm 4-0 PDO thread was placed laterally in the dermis 1 cm apart using a 25G needle and sutures were twisted in one direction 10 times. In Zone G-2, a 90mm 4-0 PDO thread was laterally placed in the dermis using a 25G needle and sutures were twisted in one direction 10 times. In Zone G-3, a 90mm 4-0 PDO thread was placed in a mesh form 0.5 cm apart in the dermis using a 25G needle, and sutures were twisted in one direction 10 times.
In Zones H and I, we assessed the differences in terms of histological changes and growth factors between threads placed in the muscle in the direction of muscle fibers and threads placed perpendicular to the muscle fibers. In Zone H-1, a 90mm 4-0 PDO thread was placed 1cm apart along muscle fibers using a 25G needle. In Zone H-2, a 90mm 4-0 PDO thread was placed 1 cm apart along muscle fibers using a 25G needle and sutures were twisted in one direction 10 times <Figure 5>.
In Zone I-1, a 30mm 4-0 PDO thread was placed 1cm apart perpendicular to muscle fibers using a 25G needle. In Zone I-2, a 30mm 4-0 PDO thread was placed 1cm apart perpendicular to muscle fibers using a 25G needle, and sutures were twisted in one direction 10 times.
[Advertisement] FCR® (Fractional Prickle CoralCalcium Regentron) – Manufacturer: (www.illglobal.com)]
Four weeks after the procedure the first pig was euthanized. We kept the pigs alive until all tissues were separated as death could change the growth factor content <Figure 6>. Before euthanasia, we used Zoletil for general anesthesia to harvest all zones. Then, we obtained 0.5 X 2cm flaps of the area between thread insertions. Flaps were sealed in pre-marked Falcon tube and flash frozen in -40℃ liquid nitrogen.
The tissues of the site of thread insertion and those in the sites of thread exit were marked with vicryl and chromic catgut, respectively. These flaps were spread evenly over a cork panels with each corner secured with a pin and fixed in formalin solution. H&E, SMA-IHC(Anti-smooth muscle actin Immunohistochemical stain), Masson-Trichrome staining were carried out and growth factor analyses were carried out using EGF·FGF·KGF·IGF·TGF-B·VEGF.
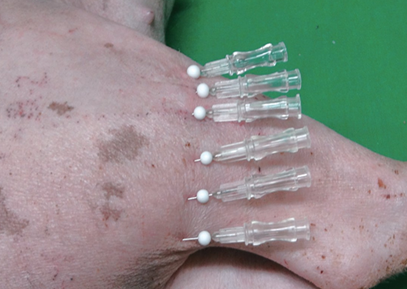
Figure 5. In Zone H-2, a 9cm 4-0 PDO thread was placed 1cm apart along the alignment of muscle fibers using 25G needle, in a simple interrupted suture technique. Sutures were then twisted in one direction 10 times
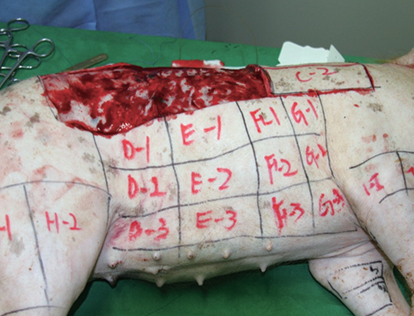
Figure 6. The first pig was euthanized 4 weeks after the procedure.
-To be continued




















