Korean meso therapy and diagnosis device manufacturer BOMTECH(www.bomtech.net) said that Digital Pro® and Digital Hand® have shown safe devices in study about ‘Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice(Annals of Dermatology, 2013 Feb)‘ participating Professor Beom Joon Kim(Department of Dermatology, Chung-Ang University College of Medicine, Korea).
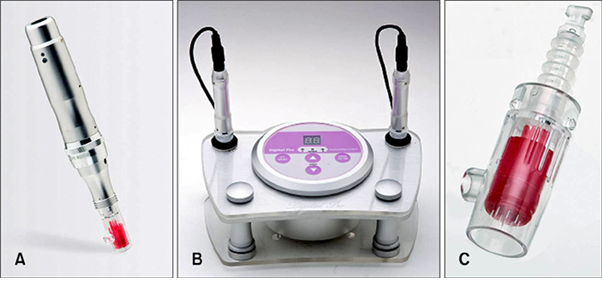
Fig. 1 Pictures of Digital Hand® and Digital Pro® (motorized microneedle devices, Bomtech Electronics Co.) showing (A) Digital Hand®; (B) Digital Pro®; and (C) a magnified picture of the microneedle portion of Digital Hand® and Digital Pro®. Therefore, when a doctor places them in contact with the patient's skin, the microneedles are automatically inserted into the skin. (Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice, Ann Dermatol. 2013 Feb;25(1):46-53.)
In this study, through the test evaluating histopathological changes, such as inflammatory cell infiltration desquamation of the stratum corneum, or disruption of the basal layer, the therapy using digital microneedle has shown safe.
DTS® using disk type-microneedle and Digital Hand® and Digital Pro® using digital microneedle perforated the skin of skin-hairless mice in this test. The test performed visual and dermatoscopic inspections, measurements of transepidermal water loss, and biopsies and compared the results function and safety of DTS®, Digital Hand®, and Digital Pro®. As the result, DTS®, Digital Hand® and Digital Pro® worked well for delivering drugs without any rejection.
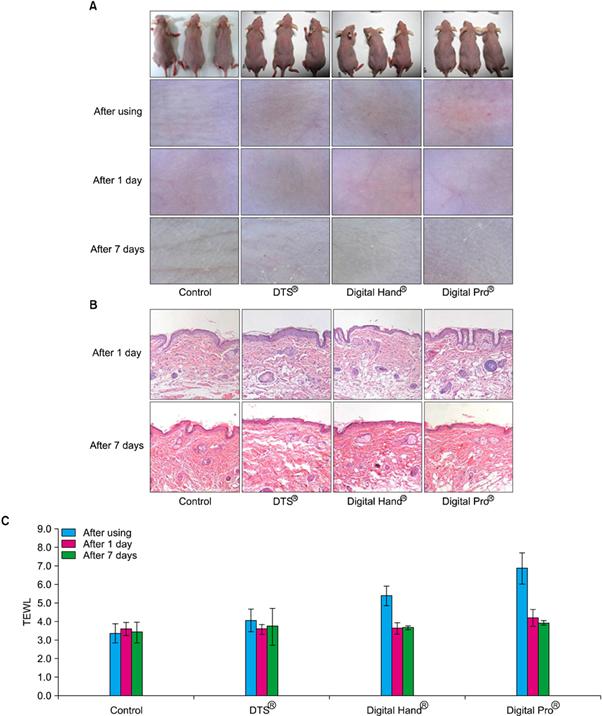
Fig. 2 Comparison of novel 1.5 mm digital microneedle devices (Digital Hand® and Digital Pro®, Bomtech Electronics Co., Ltd.) and 1.5 mm DTS® (DTS Lab.) using (A) dermatoscopic inspection, (B) histopathological examination, and (C) transepidermal water loss (TEWL) measurements.(Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice, Ann Dermatol. 2013 Feb;25(1):46-53.)
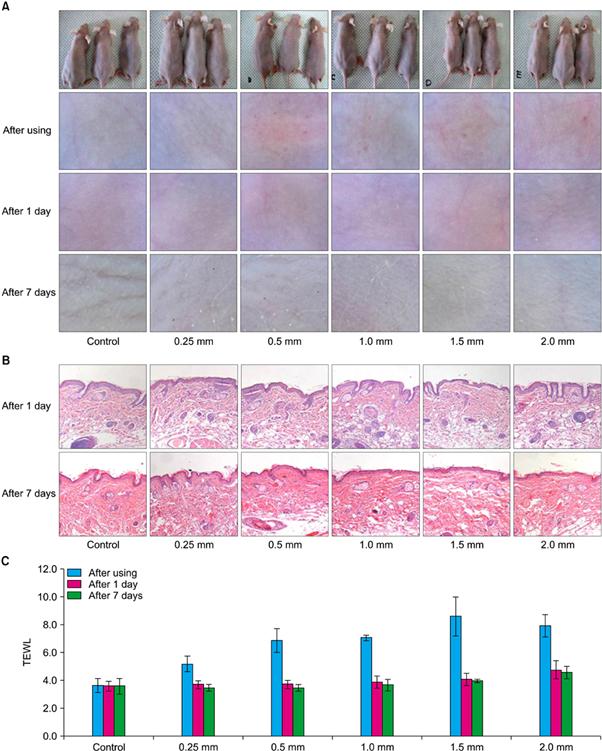
Fig. 3 Comparison of different needle sizes (no application, 0.25 mm, 0.5 mm, 1.0 mm, 1.5 mm, or 2.0 mm) using (A) dermatoscopic inspection, (B) biopsy for Digital Pro® (Bomtech Electronics Co., Ltd.), and (C) transepidermal water loss (TEWL).(Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice, Ann Dermatol. 2013 Feb;25(1):46-53.)
Digital Hand® and Digital Pro® have function of digital speed control, needle length control and accurate operation. Especially customers are attracted by these devices because of reasonable price and high-quality.
An official of BOMTECH said that “Combination medical device Digital Pro® and Digital Hand® designed by vibration free and noise free methods. These are innovative products because usage safety is higher and wrist fatigability is minimized so customers are very satisfied”.
■ Read the Study : Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice(Ann Dermatol. 2013 Feb;25(1):46-53.)
by Anna Shin(www.facebook.com/anna.shin.3705), D&PS



















