
Following reports of its excellent efficacy in skin regeneration and tightening, High-Intensity Focused Ultrasound(HIFU) devices succeeded in commercialization and are used widely in minimally invasive dermatologic procedures. Commercialization of aesthetic HIFU devices were easier thanks to the already established safety and efficacy profiles of surgical HIFU used in stone removal or cancer therapy. Continuous evaluation of safety and reliable performance is needed due to the inconsistency inherent in HIFU.
Based on the available scientific understanding of ultrasound used in various therapies, the Ministry of Food and Drug Safety(MFDS) of Korea recognized the vast potential of HIFU, such as the benefits of quick recovery and minimal side effects of HIFU-assisted removal of tumor tissues. With wider usage, the need for proven safety of direct thermal impact on human tissues is growing. Therefore, I would like to provide some help to preparing guidelines for HIFU device assessment to facilitate safe use of devices and development of related products.
Commercialization in the field of dermatology was very successful in the past 2-3 years. Five HIFU devices including Wontech’s Ultra Skin are currently used in clinical practice. This was possible due to the MFDS’s rapid and extensive evaluation of safety, performance and efficacy which led to approval of new devices. HIFU device used in surgery is categorized under Middle Category A35000 ‘Electronic Surgical Equipment’ and Small Category A35100.02, Grade 3 according to MFDS Notification No.2014-1 ‘Regulations regarding medical device category and grades(revised January 6, 2014).
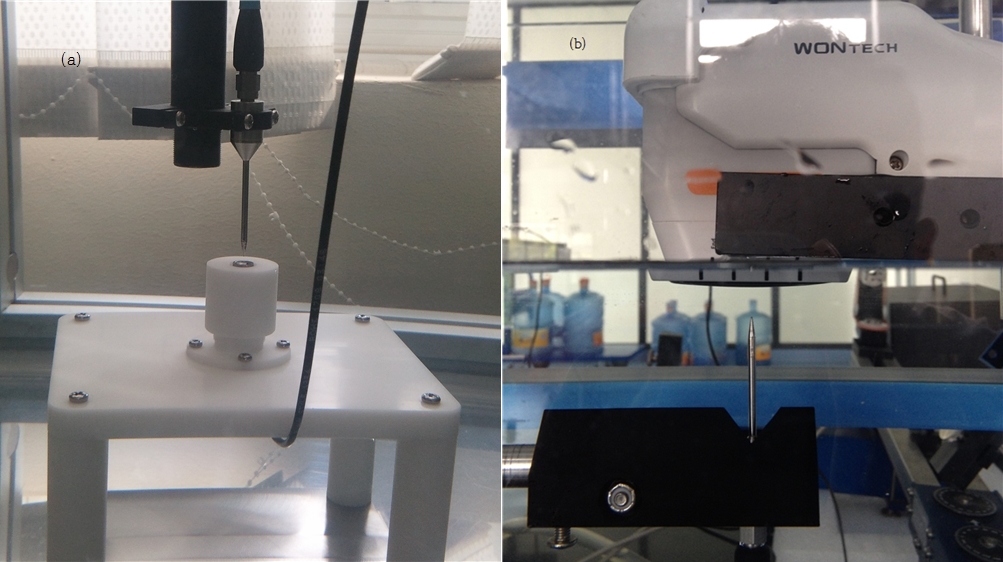
Figure 1. Measurement of HIFU wavelengths using the three-axis control system.
The three axis control system and a hydrophone are needed to assess the performance of a HIFU device. An ultrasound phantom is used for analyzing the energy emitted from the HIFU transducer. An infrared camera that accurately measures the temperature is beneficial. However, extensive knowhow is required to prepare and operate these devices. Proper evaluation of the quality and performance are possible after trial and error.
For example, to measure the wavelength range of a HIFU transducer with the focal distance of 4mm and thermal coagulation point diameter of 2-4mm, the three axis control system moves the pointy part of the hydrophone(point of measurement) over the appropriately placed transducer. This setting is shown in <Figure 1 a>. It is important to reduce measurement error by allowing micro movement of the apparatus. The finished product has to go through a final wavelength range assessment with a cartridge device as shown in <Figure 1 b>.
The second most important performance test measures the output. A force balance meter is needed to measure the output. The force balance meter resembles a scale and accurately measures the output of a HIFU transducer. As shown in <Figure 2>, an aluminum cone is placed in the center of a water container equipped with a sound absorbing element and a two-axis carrier is used to place the transducer over the sharp end of the cone. Using zero point adjustment, accurate measurements can be obtained. The most important aspect of measurement is the sturdiness of the measuring zig as it needs to produce consistent values despite repeated attachment and removal.

Figure 2. Force balance meter for measuring output.
[Advertisement] Ultra Skin/Pastelle – Manufacturer: WONTECH(www.wtlaser.com)
-To be continued-




















